What you need:
- 5-10 Australian coins of the same value (pictured here are $1 coins, but any round coins will do)
- Cardboard, paper towel, a pencil and scissors
- 5-10 zinc-plated metal washers (similar size to the coins)
- Cup or small bowl of water
- A few teaspoons of salt and a teaspoon
- A splash of white vinegar
- Red LED (light emitting diode, from electronics store)
What to do:
- Trace around a coin to make 10 circles on the cardboard and use the scissors to cut out the cardboard circles.
- To the cup or bowl of water, stir in one teaspoonful of salt at a time until no more salt will dissolve.
- Add a splash of vinegar to the salty water.
- Place the circles of cardboard in the salty water and vinegar mixture and leave them to soak for 15 minutes.
- Use the spoon to remove 5 cardboard circles from the salty water and place them on a piece of paper towel. The cardboard circles must be wet, but not dripping.
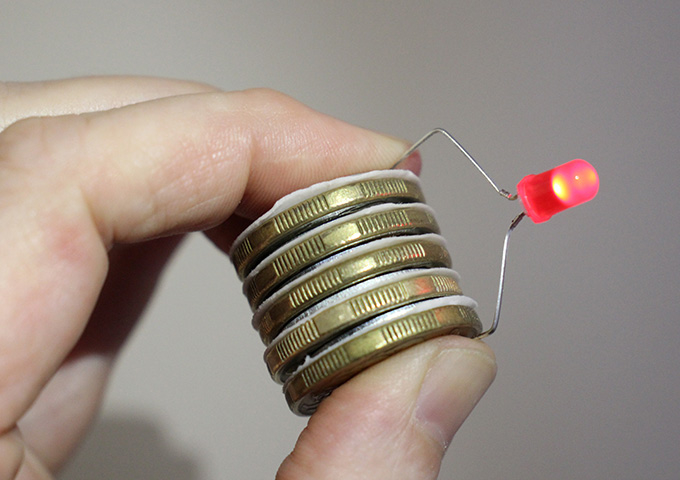
- Stack the items in the following order: coin, cardboard, washer. Repeat this pattern—end with a washer on top.
- The longest leg of the LED is the positive side and must be connected to the coin at the bottom of the stack. Carefully bend the legs of the LED so the positive leg can be held against the coin at the bottom, and the negative leg can be held against the washer at the top, as shown in the picture. The LED should light up! (If it doesn’t work, check the order of the stack, check the cardboard is still wet, and check that the legs of the LED are only touching the bottom coin and top washer in the stack).
- Try stacking 10 coins, 10 pieces of cardboard and 10 washers. Hold the stack with the thumb and finger of one hand and you might feel a buzz of electricity in your fingertips.
What’s happening?
You have created a wet cell battery, with each group of coin, cardboard and washer forming one cell. Australian coins consist mostly of copper (Cu), so the coins act as copper electrodes. The zinc-plated washers are the zinc (Zn) electrodes in the battery. The salty water and vinegar solution acts as an electrolyte, allowing electrical charge to move between the zinc and copper electrodes, creating an electrical potential across each ‘cell’.
The wet cell battery pictured here generated 0.8 volts of electricity per cell and a total of 4 volts across the five cells.






